References
1. Watanabe J, Muro K, Shitara K, et al. JAMA. 2023;329:1271-1282 2.
Vectibix® (panitumumab) prescribing information, Amgen. 3. Douillard J-Y, Oliner KS,
Siena S, et al. N Engl J Med. 2013;369:1023-1034. 4. AVASTIN® (bevacizumab)
prescribing information, Genentech. 5. Ma P, Yang BB, Wang YM, et al. J Clin Pharmacol.
2009;49:1142-1156. 6. Peeters M, Price T, Taieb J, et al. Br J Cancer. 2018;119:303-312.
7. Muro K, Watanabe J, Shitara K, et al. Poster presented at: Annual Meeting of the European
Society of Medical Oncology (ESMO); September, 2022; Paris, France. 8.Yoshino, T. Presented at:
2022 ASCO Annual Meeting; June 3-7, 2022; Chicago, IL. 9. Chen D, Li L, Zhang X, et al. Medicine
(Baltimore). 2018;97:e0097. 10. Arnold D, Lueza B, Douillard
JY, et al. Ann Oncol. 2017;28:1713-1729. 11. Tejpar S, Stintzing S, Ciardiello F, et al.
JAMA Oncol. 2017;3;194-201. 12. Loree JM, Dowers A, Tu D, et al. Clin Cancer Res.
2021;27:52-59. 13. Kobayashi Y, Komatsu Y, Yuki S, et al. Future Oncol. 2015;11:617-627.





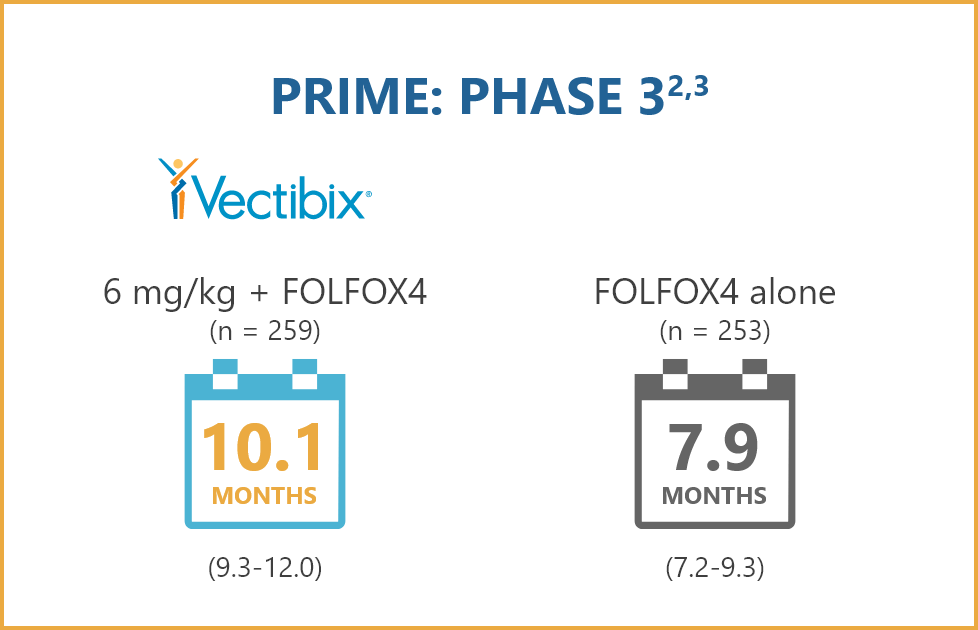
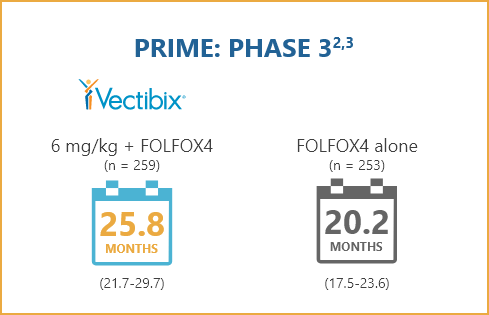

 STUDY POPULATION
STUDY POPULATION
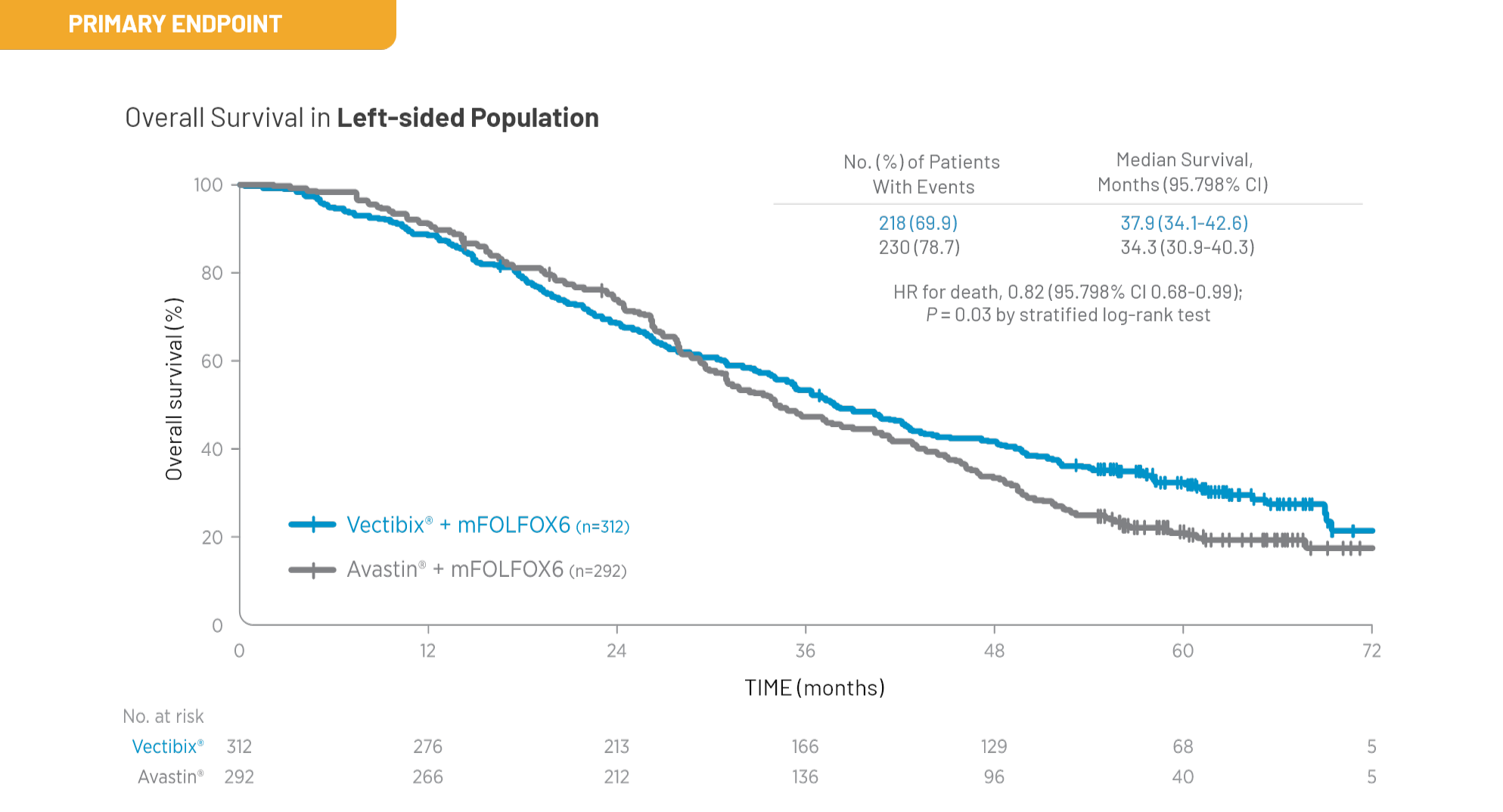
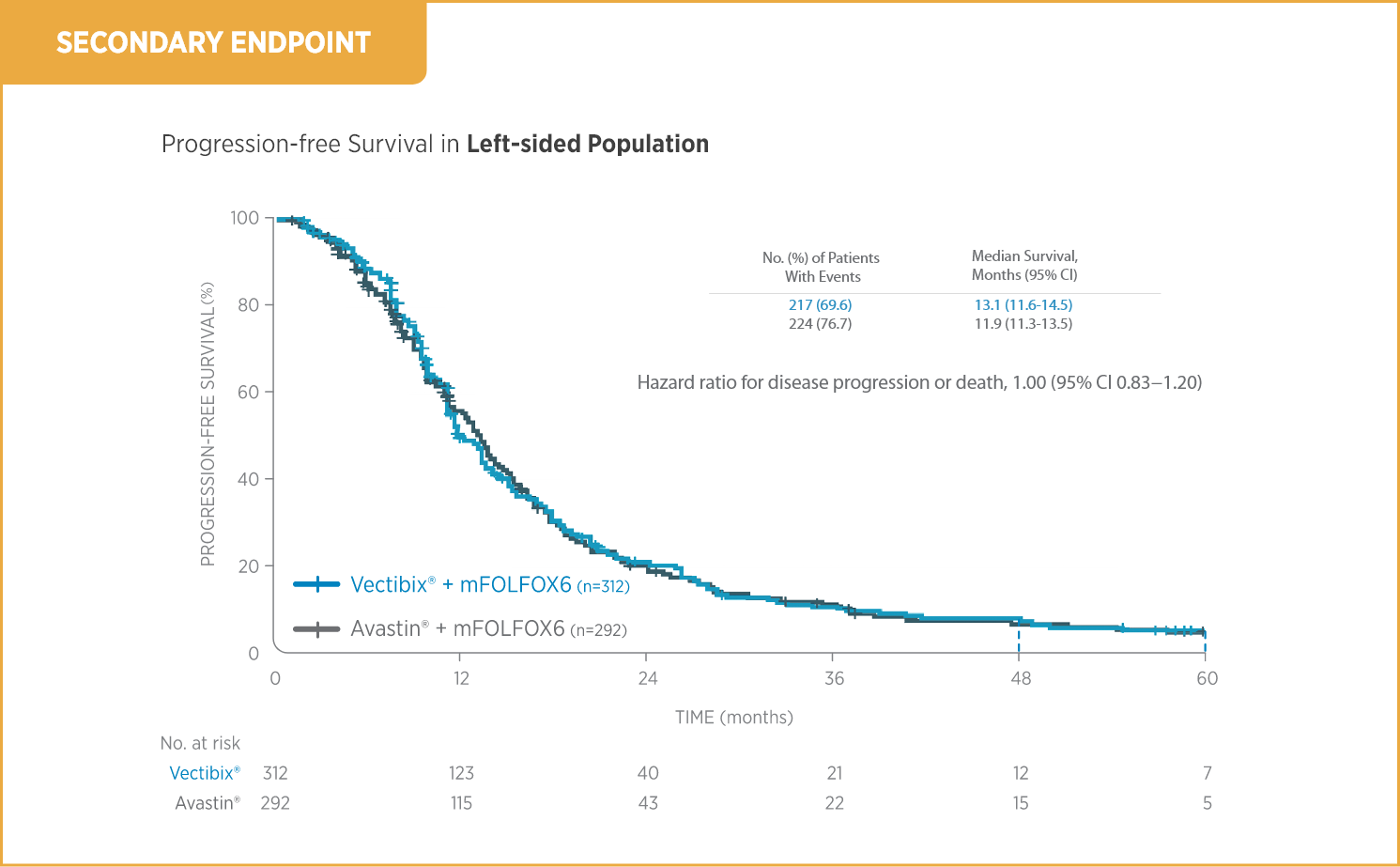
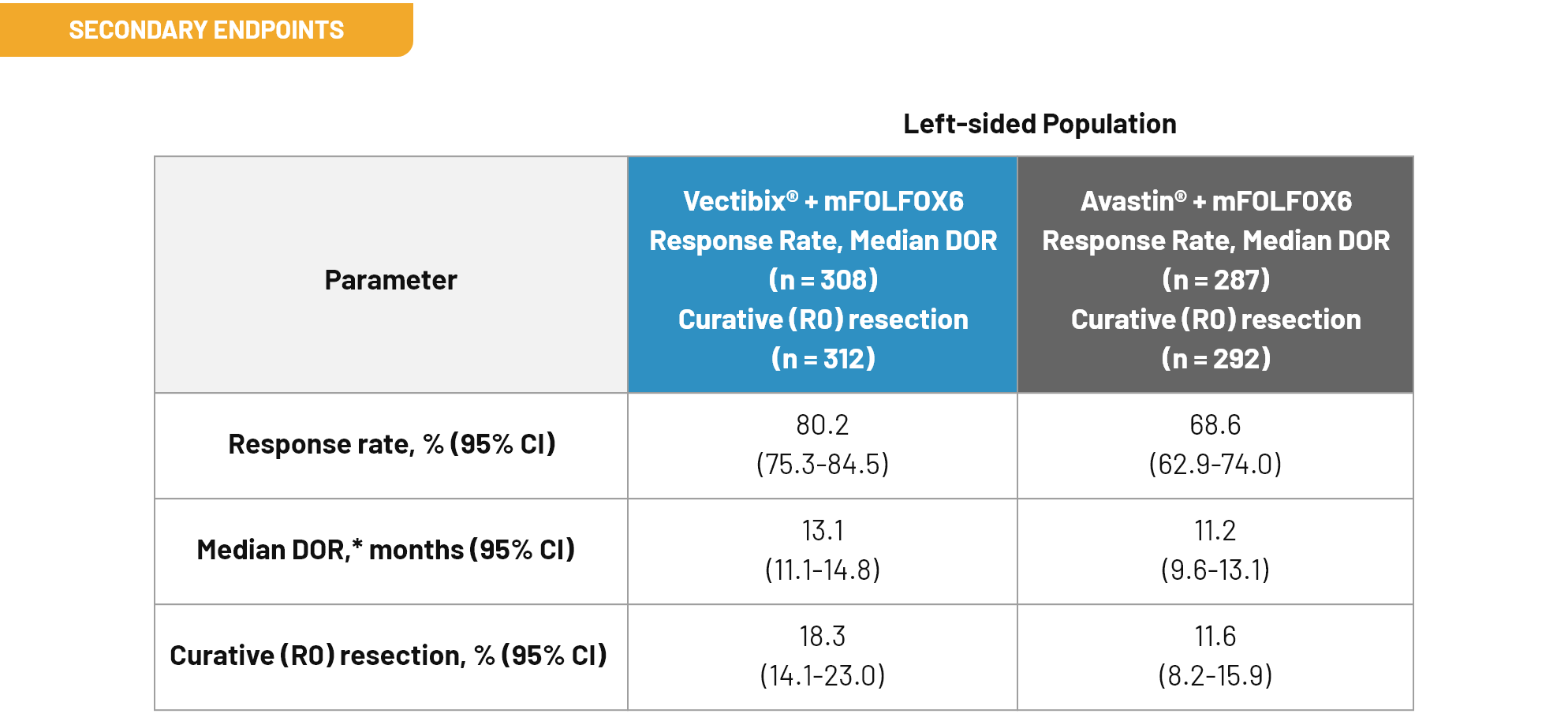
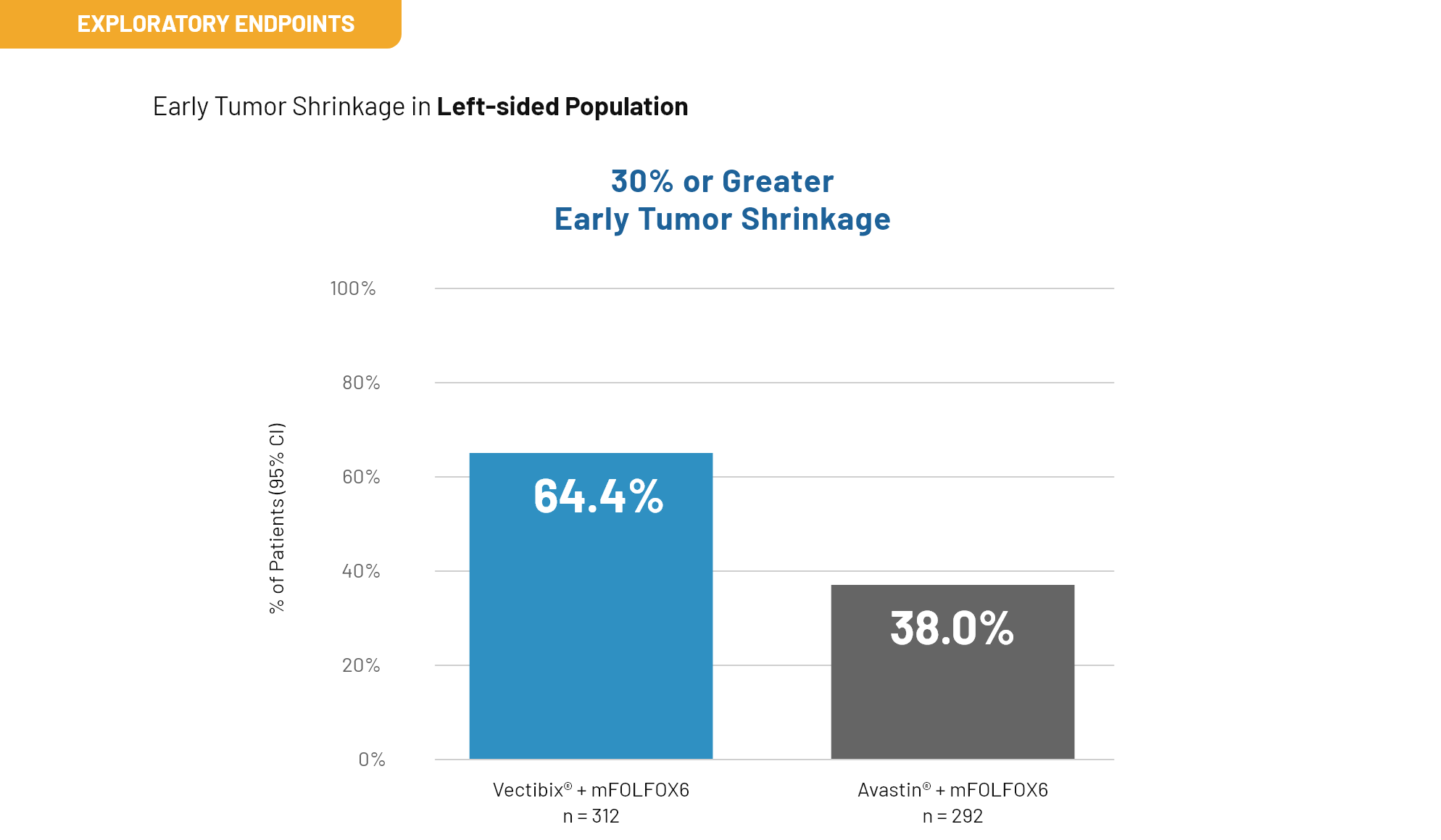
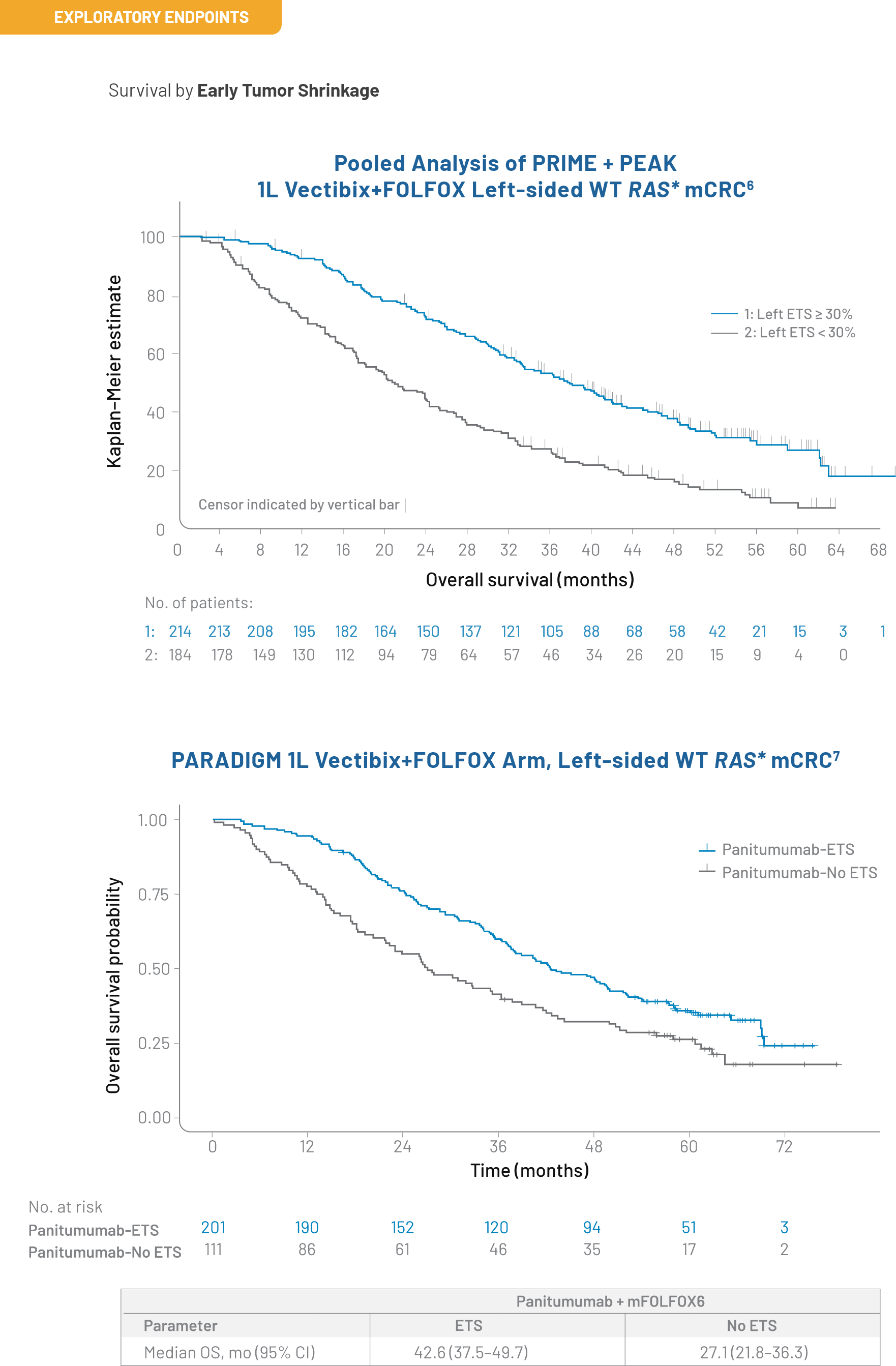

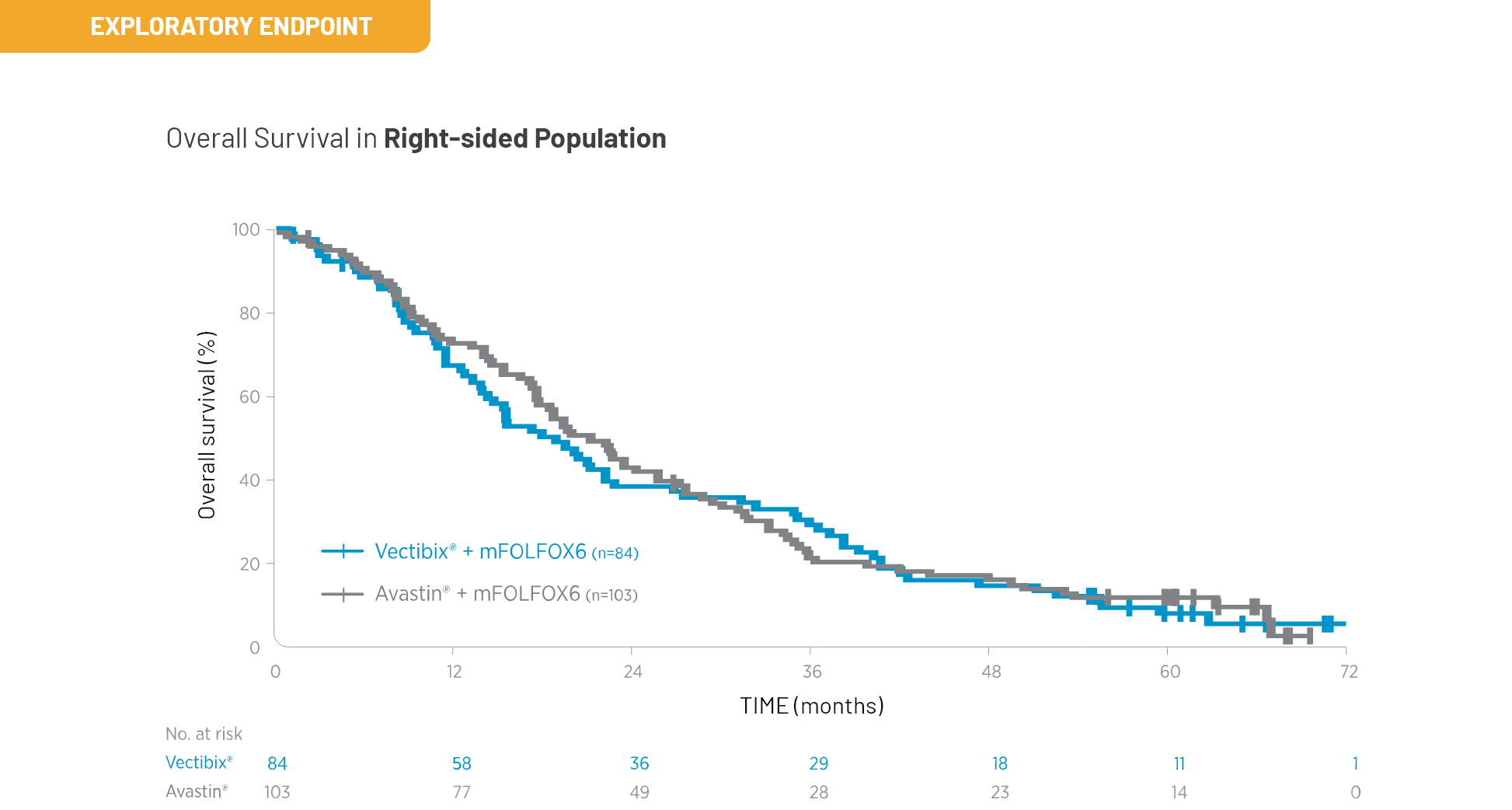
 Outcomes
Outcomes

