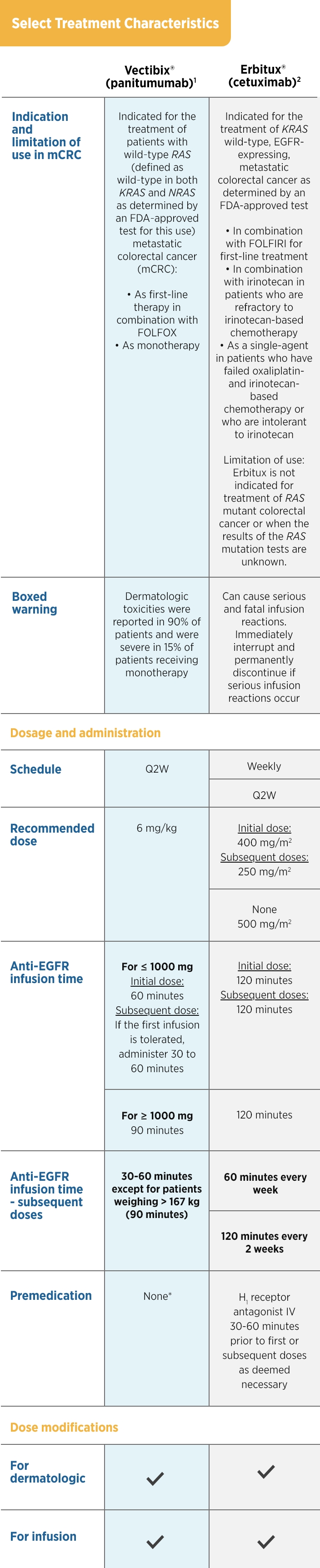
ASPECCT STUDY DESIGN
A phase 3, randomized (1:1), open-label, non-inferiority study of 1,010 patients with chemorefractory WT KRAS* mCRC treated with Vectibix® (n = 499) or cetuximab (n = 500).1,3,† Patients received prior treatment with irinotecan, oxaliplatin, and a thymidylate synthase inhibitor.
- Primary endpoint was OS assessed for non-inferiority1
- Key secondary endpoints were PFS, ORR,‡ and safety3
| |
Vectibix® |
Cetuximab |
| Treatment Characteristics |
| Schedule |
Q2W |
QW |
Dose
(administration time) |
6 mg/kg (60 min)
(Doses of > 1,000 mg should be
administered
over 90 min. If the first
infusion is tolerated,
the subsequent
infusions may be administered
over
30 to 60 min) |
250 mg/m2 (60 min) |
Loading dose
(administration time) |
None |
400 mg/m2 (120 min) |
| Premedication |
None§ |
H1 antagonist before infusion |
| Dose modifications |
| For dermatologic toxicity |
![]() |
![]() |
| For infusion reactions |
![]() |
![]() |
*Exon 2 in codons 12 and 13.1
Modified intent-to-treat population that included all patients who received at least one dose of therapy.1
Objective tumor response was evaluated by the investigator at each site using RECIST v1.1 criteria.3
§No standardized premedication was required in clinical trials. The utility of premedication in preventing infusion toxicity is unknown.1
ASPECCT = A Study of Panitumumab efficacy and safety Compared to Cetuximab; mCRC = metastatic colorectal cancer; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; Q2W = every 2 weeks; QW = weekly; RECIST = Response Evaluation Criteria in Solid Tumors; WT = wild type.