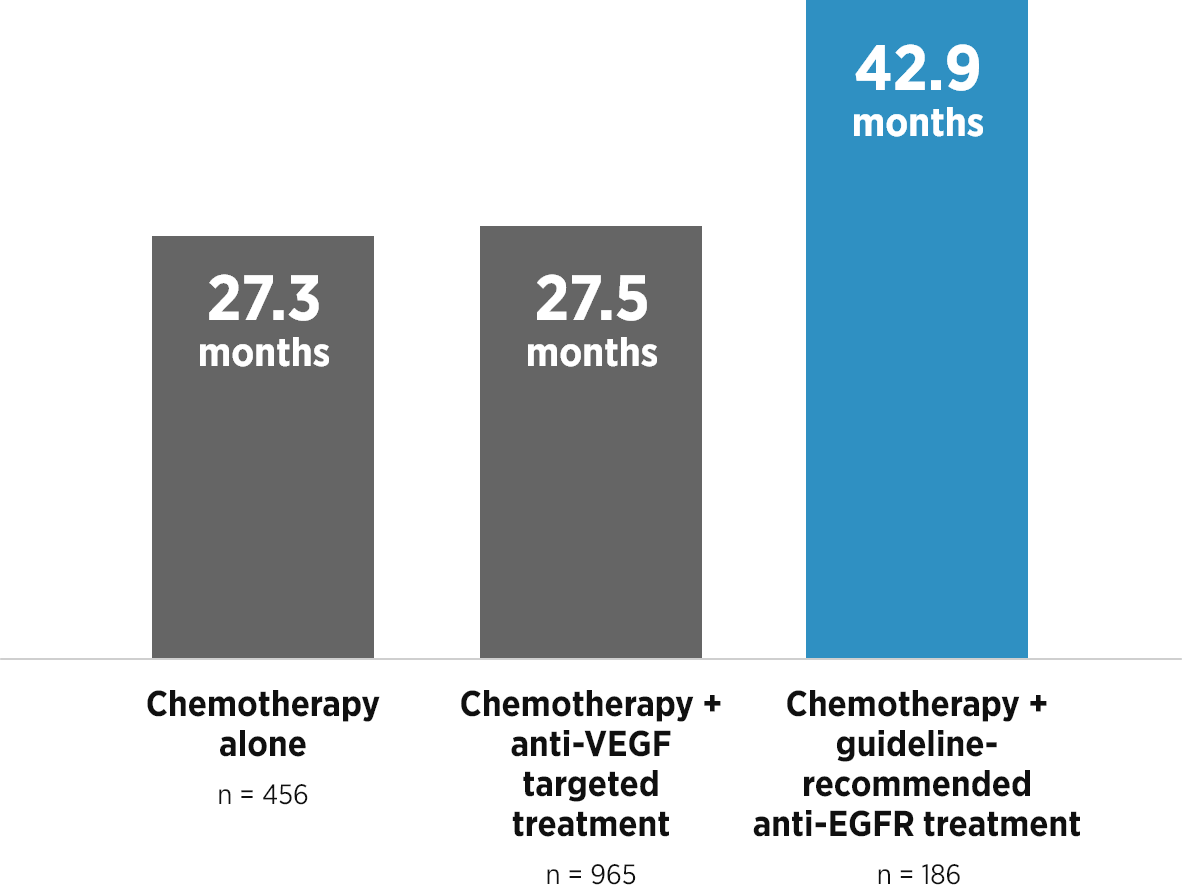
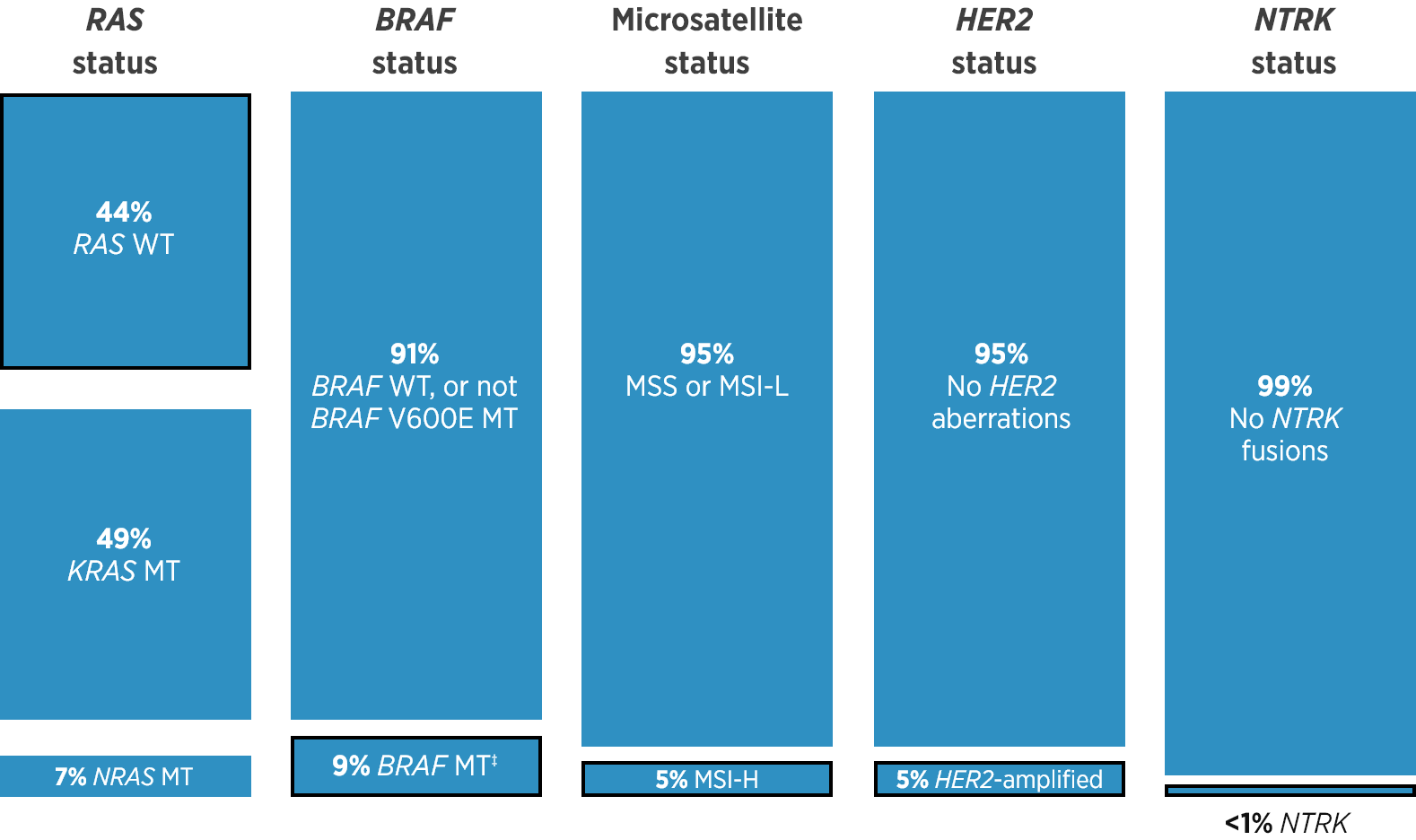
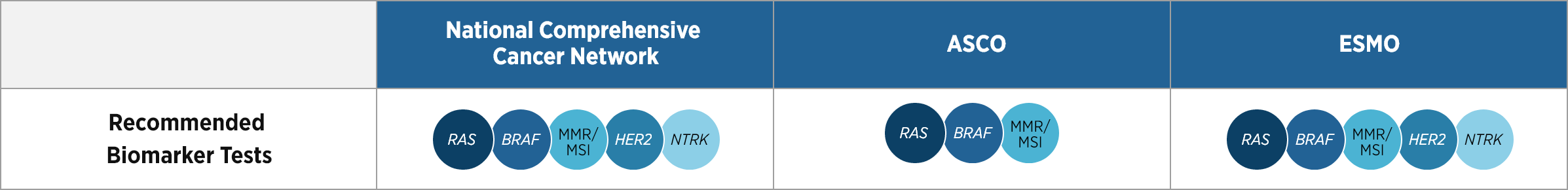
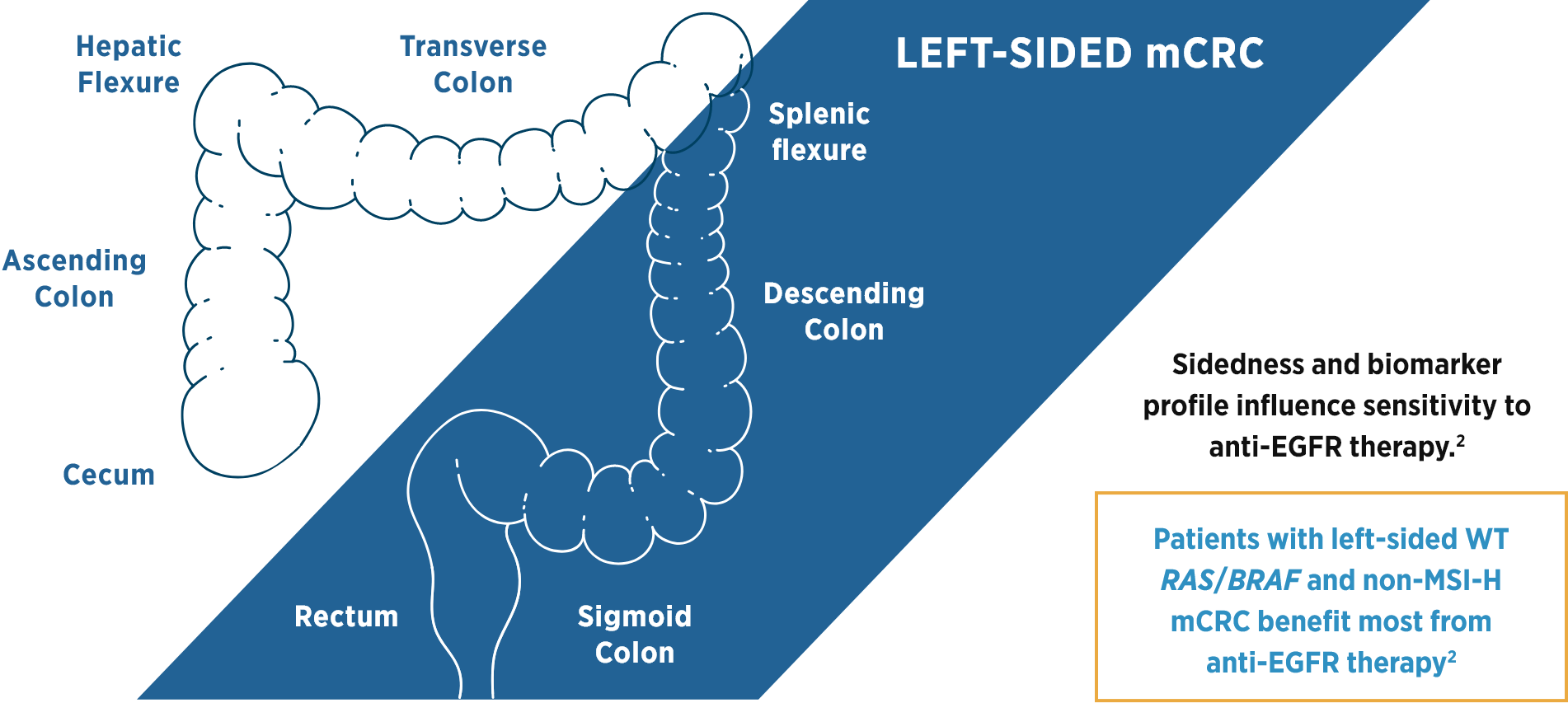
References
1. Morris VK, Kennedy EB, Baxter NN, et al. J Clin Oncol. 2023;41:678-700. 2. Doleschal B, Petzer A, Rumpold H. Front Oncol. 2022;12:1048166. 3. Cohen R, Pudlarz T, Delattre JF, Colle R, André T. Cancers (Basel). 2020;12:2350. 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer V.4.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed August 7, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. 5. Systemic therapy for metastatic colorectal cancer (mCRC) algorithm. ASCO Guidelines®. 2022. 6. Nevala-Plagemann C, Iyengar S, Trunk AD, Pappas L, Haaland B, Garrido-Laguna I. J Natl Compr Canc Netw. 2022;20(3):268-275. 7. Sepulveda AR, Hamilton SR, Allegra CJ, et al. J Clin Oncol. 2017;35:1453-1486. 8. Cervantes A, Adam R, Roselló S, et al. Ann Oncol. 2023;34:10-32. 9. Iyer P, Deng M, Handorf EA, Nakhoda S, Dotan E. JNCI Cancer Spectr. 2022;6:pkac065. 10. Vectibix® (panitumumab) prescribing information, Amgen. 11. El-Deiry WS, Goldberg RM, Lenz HJ, et al. CA Cancer J Clin. 2019;69:305-343. 12. Pennell NA, Arcila ME, Gandara DR, West H. Am Soc Clin Oncol Educ Book. 2019;39:531-542. 13. Mendis S, Beck S, Lee B, et al. Asia Pac J Clin Oncol. 2019;15:136-143.